When searching for the ideal sanitization or disinfectant product, you’ll likely come across solutions based on two “technologies”: HOCL and OCI- The challenge is in understanding which makes sense for your business. To the uninitiated, both hypochlorous acid (HOCl) and Hypochlorite (OCl-) may seem similar in composition and functionality. However, which one you use in your establishment may hold the key to effective implementation of your enhanced cleansing protocols.
Before the Verdict: Understand the Charges
As a disinfectant, Chlorine is referred to as Free Available Chlorine (FAC), and may be available as either HOCI or OCI- Technically speaking, both are used to fight germs. Before passing judgement on which of the two types of disinfectants is more effective, however, lets revise a bit of chemistry. More specifically, we’ll briefly talk about molecular charges. Notice the chemical element symbol of Hypochlorite – it has a “negative” sign at the end of it (OCI-). The symbol for hypochlorous acid does not have any sign.
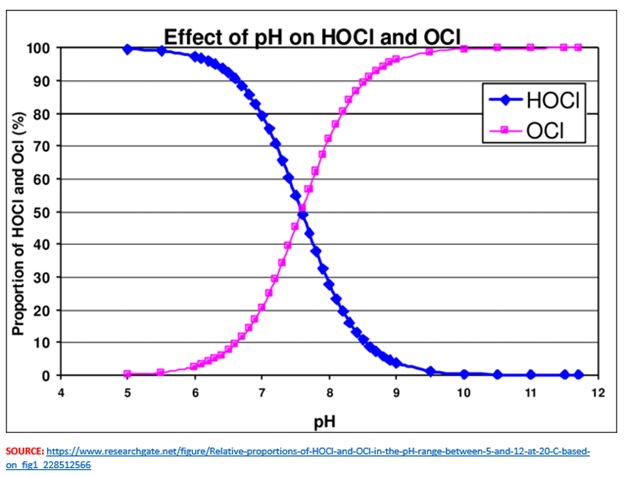
The sign (or lack of it) indicates that one type of product (OCI-) has a negative charge (pink line in the graph above), and the other (HOCI) has a positive charge (blue line). This inverse relationship increases/decreases depending on the relative acidity, based on pH levels in the water used in their manufacture.
Making Sense of the Charge
FAC is composite of both hypochlorous acid and hypochlorite ions. As manufacturers increase pH levels, the percentage of hypochlorous in their products declines, transforming the product into a hypochlorite ion heavy version of the disinfectant.
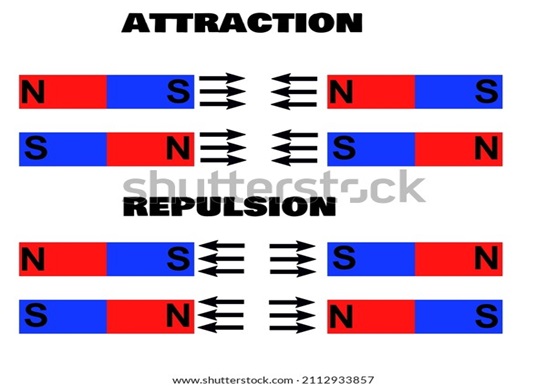
And here’s an important bit of physics to help us make sense of why a negative charge in OCI-, and an absence of a charge in HOCI matters. To understand the importance of these charges in the disinfection process, let’s remember how like poles in a magnet repel, and how unlike ones attract. A negatively-charged magnet repels another negatively-charged object. However, two dissimilar charges – negative and positive, attract. Attraction also occurs with one charged surface and another neutral (no charge) object. And there lies the power of hypochlorous acid.
Reaching a Verdict
Germ infested surfaces typically have a negative charge. Therefore, because OCI- is itself negatively charged, a hypochlorite ion heavy disinfectant causes germs to “run away” (repel) from it. Spraying any amounts of OCI- disinfectant does not accomplish the objectives of a enhanced sanitization protocol.
At the right pH levels, HOCI, on the other hand, has no charge. This allows it to trick the negatively-charged germ surfaces to accept it and absorb the disinfectant. When that happens, the disinfectant takes over and, through osmosis, neutralizes the germs. The end result, is that hypochlorous-based disinfectants adhere to germ surfaces better than their hypochlorite ion heavy counterparts.
People also often ask whether bleach-heavy products are more effective than hypochlorous-based solutions? The answer, once again, is no! Bleach-based products contains concentrated amounts of sodium hypochlorite (NaOCl-). This increases the pH levels of the resulting formulation. Concentrated bleach (at 13 pH or higher) may be an effective “germ buster”, but unlike hypochlorous acid, it is extremely unsafe to handle, and may cause adverse effects on pets, humans, and plants.
The verdict: HOCI is a more effective disinfectant than OCI-









